Covid-19 has changed how the world functions and has put life to stand still. While all of us are trying to be safe and opting to stay indoors, we are also dealing with many uncertain questions like when we will get the vaccine, will it be safe to take the vaccine, are our kids going to get the vaccine, etc. While everyone has started to take their vaccines since Phase 2 is on full swing, the questions remain unanswered.
At Kidsstoppress, we believe in simplifying parenting for you by giving you all the information we have in hand. We also believe in sharing expert advice and also share with you what other parents are opting for so you can make the best choice for your family.
Here's everything you need to know about India's biggest vaccination drive and how to register yourself as a beneficiary.
COVID – 19 Vaccine

Covid-19 vaccination phase is in full swing and senior citizens are eligible to get the vaccine. Soon the entire population will receive their first vaccine shot. The country’s top drug regulator, DCGI has approved Serum Institute Of India's COVID-19 vaccine Covishield and Bharat Biotech’s Covaxin for restricted emergency use.
Who will get the vaccine first

At first, there were only be a limited number of doses, so the government has drawn up plans to prioritise the most vulnerable people. But soon everyone will be eligible to get the dose.
The priority looks as follows:
1. Healthcare workers
2. Frontline workers
3. People aged above 50
4. Areas recording high infection of Covid-19
5. Remaining population
Concerns About The Covid-19 Vaccine

1. Which vaccine is the safest for us?
2. Concern over the rushed approval
3. How quickly could COVID-19 Vaccine stop the pandemic?
4. Will the COVID-19 vaccine show long-term effects?
5. What are the side effects of the vaccine?
6. Is it safe for my child?
7. If we don't give the vaccine to our children, how are they supposed to attend school?
8. Is the vaccine going to be mandatory?
9. Should people with severe allergies hold off the vaccine?
10. Should people who had COVID-19 before still take the vaccine?
4 questions parents are asking about the COVID-19 vaccine:
1. When can my child be vaccinated?
Clinical trials involving children are only just getting started. Since they just started trials with children ages 12-17, the vaccine’s efficacy and safety will have to be evaluated for each age group, and testing hasn’t started for infants, toddlers or kids in the U.S.
2. Will children need more shots than adults?
It does not appear that the schedule of COVID-19 vaccine doses will be different for children, but that could change as testing goes on. Pfizer’s and Moderna's vaccine is being tested in adolescents with a two-dose series, three to four weeks apart, just like in adults. The second dose serves as a “booster shot,” since the first dose alone doesn’t provide optimal immunity.
3. Are the vaccines safe for kids?
So far, no serious safety concerns have been identified with either the Pfizer or Moderna vaccines, but the trials are still in the early stages for children. One concern has been temporary side effects. Children tend to have stronger immune systems than adults, and they may have stronger temporary reactions to the vaccine. That could mean more pain and swelling at the injection site for a few days and possibly a fever.
4. Isn't vaccinating adults enough?
Just vaccinating adults would not be enough to end the pandemic. Children can still become infected, transmit the virus and develop complications. If a vaccine is not available, children will likely serve as a reservoir of the virus, making it harder to end the pandemic.
Pfizer and Moderna for children and pregnant women
Research has indicated that many children clear the coronavirus before it leads to severe disease, resulting in mild or asymptomatic cases. That means kids aren't a priority group when it comes to vaccinations. They're also difficult to include in trials because of ethical and safety concerns. Some parents may not feel comfortable signing their kids up to participate, and researchers often prefer to test vaccines in a small group of adolescents before enrolling young kids.
Pfizer's clinical trials, for instance, did not include people younger than 12. And the sample size of kids from ages 12 to 15 was too small to weigh the risks. Moderna's trial didn't include children at all. But there's little reason to suspect that kids will react poorly to the shots.
Children generally respond well to vaccines. They should respond well or comparably to a young adult – and maybe even better." – said Donna Farber, A Columbia immunologist to Business Insider.
Both the Pfizer and Moderna late-stage trials excluded pregnant people. They asked female volunteers to take a pregnancy test before each vaccination, and any with positive results were not allowed to continue.
Pfizer and Moderna plan to follow up with pregnant women who choose to get vaccinated now that the shots are authorized. That's how public-health experts eventually determined it was safe for pregnant women to get the flu vaccine, even though they were never included in the trials.
For children under 18
There is still no talk about (inculcating) those under 18 years. Even during the trials. we have conducted inculcations for those above 18. – Amit Bhatia, Director of Jeevan Rekha, which is conducting rials for Bharat Biotech’s Covaxin to Bangalore Mirror
There is no clarity if kids under 18 will get the vaccine, but the real question is if we are comfortable sending our kids to school at all? Most parents are waiting for the vaccine before kids can be back to physical school again.
“It is a wise decision not to vaccinate the kids yet because of unknown long term effects. For the school model to be successful, parents must report o the authorities if their kid is positive or primary contact. Testing needs to be ramped up. Group activities should be stopped. Schools should educate parents on prevention and their responsibilities towards keeping their children healthy so that their children can adjust to the new normal.” – Ritu Sama, a parent who spoke to Banglore Mirror
But later yesterday, Bharat Biotech, which received emergency approval for its COVID-19 vaccine only in "clinical trial mode", is allowed to conduct its trials on children who are above the age of 12 years. The vaccine has already been used for children above 12 in the last round and has been found safe. The Hyderabad-based firm is conducting Phase 3 trials.
Does the vaccine mean back to normal life?
Since there is no evidence that the immunization prevents transmission, wearing maks, social distancing and regualar handwashing are going to be the new normal.
"As with all vaccines, it may work really great in certain patient subsets, but not as well in others. Does that mean you are free to hop on to a plane or have 30 people over at your house? Probably not", Dr. Michelle Barron, senior medical director for infection prevention at Colorado's UC Health.
How to register for vaccination?
Common Service centres need to be utilized for self-registration and identity certification of the general population. Individuals can select the method of authentication from the following method
Biometric: With this demographic details of the individual from name to a permanent address in Aadhaar card will autofill the platform.
OTP Authentication: An OTP will be sent to the registered mobile number with an Aadhaar card. If the OTP authentication is successful, demographic details of the individual as per the Aadhaar will auto-populate.
Demographic Authentication: An individual can also enter all demographic details manually i.e. Name, DoB, gender and select Demo Authentication. A green tick will appear confirming the same.
An individual interested to register will be required to provide photo identity from one of the following:
- Aadhaar Card
- Driving License
- Health Insurance Smart Card issued under the scheme of Ministry of Labour
- MNREGA Job Card
- Official identity cards issued to MPs/MLAs/MLCs
- PAN Card
- Passbook issued by Bank/Post Office
- Passport
- Pension Document
- Service Identity Card issued to employees by Central/State Govt./PSUs/ Public Limited Companies
- Smart Card issued by RGI under NPR
- Voter ID
- Photo identity can either be uploaded on Co-WIN system (in PDF, JPG or PNG file formats) or can be pulled from the existing Digi Locker account of the individual
- Once registered, date and time will be allocated for vaccination. There will be no spot registration facility and only pre-registered beneficiaries will be allowed to proceed with vaccination.
District Collector (DC)/District Magistrate (DM) with the support of District Immunization Officer will link the sessions sites, vaccinators, supervisors and beneficiaries and decide the dates and time for conducting the vaccination session. The respective district administration will approve the beneficiaries for session and site allocation. Co-WIN has inbuilt monitoring and reporting mechanism.
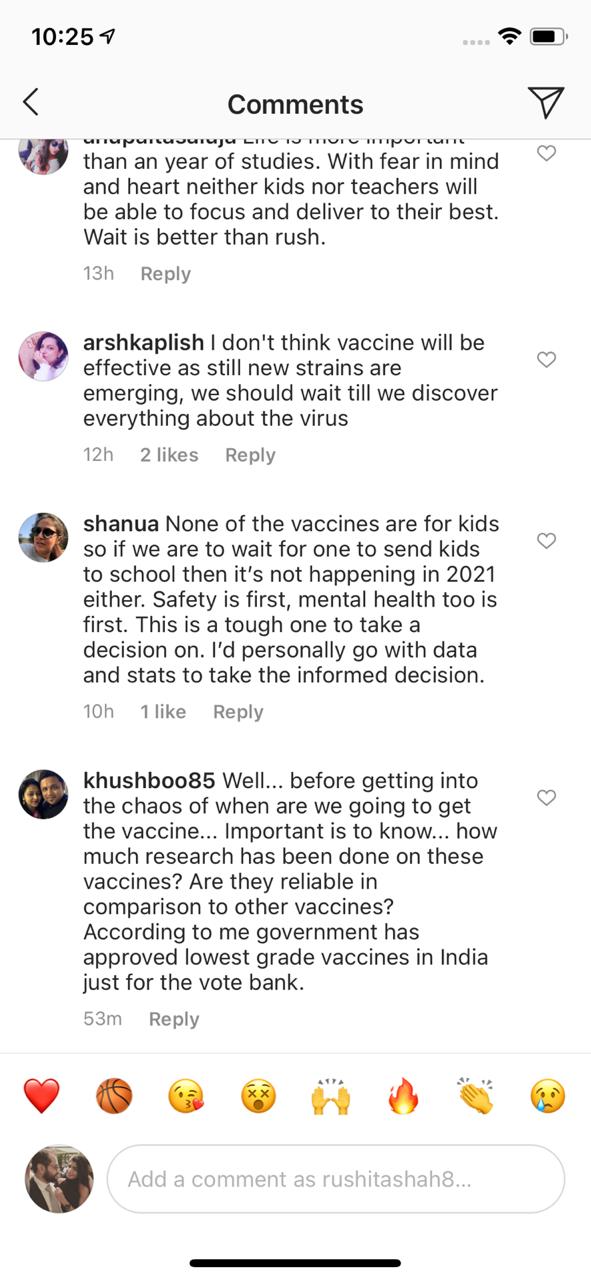
So, parents' COVID-19 vaccines are ready. Are you? We asked this question and here's what some of them said.
Here's what everyone responded

Write to us what your concerns are at [email protected]